United Therapeutics Corporation History
Please see Important Safety Information for our FDA approved products under the Research and Medicine tab.
2024
First successful transplant of a porcine xenothymokidney
Read the Press Release
First successful transplant of a xenokidney from a pig with ten gene edits 500th lung transplant using UT’s EVLP service
Read the Press Release
2023
United Therapeutics and Miromatrix Medical announce completion of tender offer and merger
Read the Press Release
United Therapeutics acquires IVIVA Medical Inc.
United Therapeutics Corporation Annual Report
View 2023 Report
2022
First successful xenotransplantation of porcine heart
Read the Press Release
Tyvaso DPI® (treprostinil) inhalation powder approved by the FDA for the treatment of PAH and PH-ILD to improve exercise capacity
Read the Press Release
Visit Tyvaso DPI page
United Therapeutics Corporation Annual Report
View 2022 Report
2021
INCREASE results published in New England Journal of Medicine
Read the Press Release
Commercial launch of the Remunity® Pump for Remodulin®
Read the Press Release
FDA approval and launch of Tyvaso® for the treatment of PH-ILD
Read the Press Release
UT becomes a public benefit corporation
Read the Press Release
United Therapeutics Corporation Annual Report
View 2021 Report
2020
FDA clearance of pharmacy fill Remunity® Pump for Remodulin®
Read the Press Release
Visit Remunity Website
UT issues first Corporate Responsibility Report
Read the Press Release

INCREASE study of Tyvaso® in PH-ILD meets primary endpoint
Read the Press Release
UT launches TETON study in IPF
Read the Press Release
United Therapeutics Corporation Annual Report
View 2020 Report
2019
UT in-licenses Ralinepag an oral, IP receptor agonist being developed for PAH
Read the Press Release
Orenitram® label expansion and relaunch
Read the Press Release
United Therapeutics Corporation Annual Report
View 2019 Report
2018
FREEDOM-EV study of Orenitram® meets primary endpoint
Read the Press Release
UT launches collaboration with MannKind for Tyvaso DPI®
Read the Press Release
Health Canada approves Unituxin®
Read the Press Release
United Therapeutics Corporation Annual Report
View 2018 Report
2017
UT's subsidiary, Lung Biotechnology PBC, launches organ bioprinting program in collaboration with 3D Systems
Visit Lung Biotechnology Website
FDA approves TD-300 Nebulizer for Tyvaso®
Read the Press Release
United Therapeutics Corporation Annual Report
View 2017 Report
2016
Launch of Unither 2.0, Major Pipeline Expansion
UT obtains $1 billion unsecured line of credit
United Therapeutics Corporation Annual Report
View 2016 Report
2015
Unituxin® approved by FDA to treat high-risk neuroblastoma
Read the Press Release
Visit Unituxin Website
UT enters into collaboration with Mayo Clinic to build and operate an ex-vivo lung perfusion center in Jacksonville, Florida
Read the Press Release
United Therapeutics Corporation Annual Report
View 2015 Report
2014
Japanese regulators approve Remodulin® for PAH under the brand name Treprost™
Read the Press Release
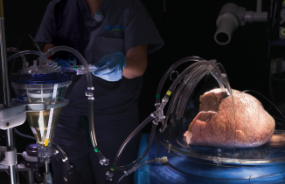
First ex-vivo lung perfusion procedure performed by Lung Bioengineering, a subsidiary of UT
Visit Lung Bioengineering website
UT opens Regenerative Medicine Lab in Research Triangle Park, North Carolina, with a focus on manufacturing lungs and lung lobes for transplant
UT and DEKA Research & Development Corp. enter into an agreement to develop a next-generation subcutaneous pump for Remodulin®, which ultimately resulted in the Remunity® Pump for Remodulin®
Read the Press Release
United Therapeutics Corporation Annual Report
View 2014 Report
2013
Orenitram® approved by FDA for the treatment of PAH to improve exercise capacity
Read the Press Release
Visit Orenitram Website
UT annual revenues surpass $1 billion
United Therapeutics Corporation Annual Report
View 2013 Report
2011
FREEDOM-M study of Orenitram® meets primary endpoint
Read the Press Release
Intravenous Remodulin® approved in most of E.U.
Read the Press Release
Adcirca® becomes most prescribed drug for PAH
United Therapeutics acquires Revivicor xenotransplantation program
United Therapeutics Corporation Annual Report
View 2011 Report
Annual Report Soundtrack
01 – “Dear Shareholders” performed by the Alan Scott Band
02 – “Live it up” performed by the Alan Scott Band
03 – “2011” performed by the Alan Scott Band
04 – “Helicopter” performed by the Alan Scott Band
2010
United Therapeutics launches collaboration with National Cancer Institute to develop a monoclonal antibody for treatment of high-risk neuroblastoma, which is today known as Unituxin®
United Therapeutics Corporation Annual Report
View 2010 Report
2009
UT surpasses $1 billion in assets
Adcirca® approved by FDA for the treatment of PAH to improve exercise capacity
Read the Press Release
Visit Adcirca Website
Tyvaso® approved by FDA for the treatment of PAH to improve exercise capacity
Read the Press Release
Visit Tyvaso Website
United Therapeutics Corporation Annual Report
View 2009 Report
2008
UT in-licenses U.S. commercialization rights to Adcirca® for PAH from Eli Lilly and Company
Read the Press Release
United Therapeutics Corporation Annual Report
View 2008 Report
2007
TRIUMPH-1 study of inhaled treprostinil meets primary endpoint
Read the Press Release
United Therapeutics Corporation Annual Report
View 2007 Report
2006
FDA approves expansion of Remodulin® label to include transitions from Flolan®
Read the Press Release
United Therapeutics Corporation Annual Report
View 2006 Report
2005
Remodulin®® approved by 23 countries in the European Union for the treatment of PAH to improve exercise capacity by subcutaneous infusion
Read the Press Release
United Therapeutics Corporation Annual Report
View 2005 Report
2004
FDA approves expansion of Remodulin® label to include treatment of PAH by intravenous infusion
Read the Press Release
United Therapeutics Corporation Annual Report
View 2004 Report
2003
UT commences first human clinical trial of an oral formulation of treprostinil
United Therapeutics Corporation Annual Report
View 2003 Report
2002
Remodulin® approved in the United States, Canada and Israel to treat PAH by subcutaneous infusion
Visit Remodulin Website
United Therapeutics Corporation Annual Report
View 2002 Report
2001
United Therapeutics Corporation Annual Report
View 2001 Report
2000
UT submits new drug application to FDA for Remodulin® to treat pulmonary arterial hypertension
1999
United Therapeutics acquires rights to treprostinil from Glaxo Wellcome Inc.
United Therapeutics acquires Synquest, Inc., providing in-house capacity and expertise to manufacture treprostinil
1997
Initial public offering (Nasdaq:UTHR), priced at $12.00 per share
1996
United Therapeutics founded by Martine Rothblatt to find a cure for her daughter's life- threatening condition, now known as pulmonary arterial hypertension